GM501 GentleVit
GM501 GentleVit Freeze/GentleVit Thaw are a set of ready-to-use media for vitrification and warming of human oocytes and embryos.
Up to 5 vitrification cycles (of the same patient) can be performed with one media set-up. Do not use the same media for different patients!
Rev03_00
| Product description | Product code | Unit |
|---|---|---|
| GM501 GentleVit Freeze Kit | 4 GVF_KIT1 | Material included in the package: |
| 1 x 5 ml GentleVit Pre-vitrification Medium | ||
| 1 x 1 ml GentleVit Freeze Medium 1 | ||
| 1 x 1 ml GentleVit Freeze Medium 2 | ||
| 1 x 1 ml GentleVit Freeze Medium 3 | ||
| 1 x 1 ml GentleVit Freeze Medium 4 | ||
| 1 x 1,5 ml GentleVit Freeze Medium 5 | ||
| GM501 GentleVit Thaw Kit | 4 GVT_KIT1 | Material included in the package: |
| 1 x 7,5 ml GentleVit Thaw Medium 1 | ||
| 1 x 1 ml GentleVit Thaw Medium 2 | ||
| 1 x 1 ml GentleVit Thaw Medium 3 | ||
| 1 x 1 ml GentleVit Thaw Medium 4 | ||
| 1 x 1 ml GentleVit Thaw Medium 5 | ||
| 1 x 1 ml GentleVit Thaw Medium 6 |
Product facts and notices
- Composition:
- Product spectifications
- Vitrification and thawing of Oocytes
- Vitrification and thawing of Embryos
- Precautions and warnings
- Storage instructions and stability
Composition:
- GM501 GentleVit Freeze/GentleVit Thaw are HTF-based and contain HEPES, sucrose, human serum albumin (12-20 g/liter). Cooling media also contain DMSO, Ethylene Glycol (EG) and Ficoll.
- GM501 GentleVit Freeze/GentleVitThaw not contains antibiotics.
Product specifications and quality control:
- All raw materials are of highest available purity (European Pharmacopoeia and/or USP standard) if applicable.
A certificate of analysis is available for each batch upon request from our website with respective lot number. - The MSDS for the media are available upon request and can also be downloaded from our website.
- GM501 GentleVit Freeze/Thaw are manufactured and tested according to the following specifications:
pH: 7.20-7.50 (release criteria: 7.20-7.40)
Osmolality (mOsm/kg):
- 270-295 (GPI/GT6: release criteria: 270-290)
- 805-865 (GT3: release criteria: 805-850)
- 535-565 (GT4)
- 405-435 (GT5)
Sterility: sterile – SAL10-3 (Sterility Assurance Level)
Endotoxins (EU/ml): < 0.25
MEA: ≥80 (1-cell assay, blastocysts after 96 h in %)
Vitrification of Oocytes:
Preparation for Vitrification
- Ensure that all media bottles of the kit are well mixed before use and warmed to room temperature (20-25°C). We strongly advice to read through all the steps of the vitrification/warming procedure before starting the procedure.
Preliminary steps:
- In a 6-well dish, fill the first well with 250-300 μl of GentleVit Pre-vitrification medium, the second with Freeze Medium 1, the third with Freeze Medium 2 and continue doing so until Freeze Medium 5.
- Open the necessary number of vitrification devices, taking into account that 1 device can hold 2-3 oocytes, in a maximum volume load of 1μl (check the instructions for the device you are using). Conveniently place the separate parts of the device on the workbench for easy access later in the procedure.
Vitrification protocol:
- Oocytes are sequentially exposed to the following media:
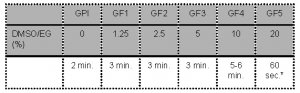
* Note: The complete process of placing the oocyte in “Freeze Medium 5”, loading the oocyte on the vitrification device in maximum 1μl GF5, inserting the device in the outer straw and sealing should not take longer than 60 seconds before plunging the device into the liquid nitrogen.
Thawing of Oocytes:
Preparation for Thawing
- Ensure that all media bottles of the kit are well mixed before use and warmed to room temperature (20-25°C). One exception is Warming 1, which must be warmed to 37°C before use. We strongly advice to read through all the steps of the vitrification/warming procedure before starting the procedure.
Preliminary steps:
- Thawing procedure: In a 6-well dish, fill the first well with 500-800μl of Thaw Medium 1, the second with Thaw Medium 2, the third with Thaw Medium 3, and continue doing so until Thaw Medium 6.
Thawing protocol:
- In the first warming step, ensure homogeneous temperature by swirling the straw gently in GT1.
- Oocytes are sequentially exposed to the following media:
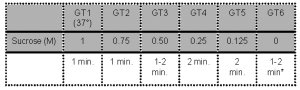
*Note: Wash for 1-2 min before transfer to culture medium.
Vitrification of Embryos (Zygote to Blastocyst):
Preparation for Vitrification
- Ensure that all media bottles of the kit are well mixed before use and warmed to room temperature (20-25°C). We strongly advice to read through all the steps of the vitrification/warming procedure before starting the procedure.
Preliminary steps:
- For vitrification of embryos, the following media are NOT required:
GentleVit Freeze Medium 1
GentleVit Freeze Medium 2
- In a 6-well dish, fill the first well with 250-300μl of GentleVit Pre-vitrification medium, the second with Freeze Medium 3, the third with Freeze Medium 4 and the last with Freeze Medium 5.
- Open the necessary number of vitrification devices, taking into account that 1 device can hold 1-2 embryos in a maximum volume load of 1μl (check the instructions for the device you are using). Conveniently place the separate parts of the device on the workbench for easy access later in the procedure.
Vitrification protocol:
- Embryos are sequentially exposed to the following media:
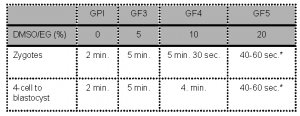
* Note: The complete process of placing the oocyte in “Freeze Medium 5”, loading the oocyte on the vitrification device in maximum 1μl GF5, inserting the device in the outer straw and sealing should not take longer than 60 seconds before plunging the device into the liquid nitrogen.
Thawing of Embryos (Zygote to Blastocyst):
Preparation for Thawing
- Ensure that all media bottles of the kit are well mixed before use and warmed to room temperature (20-25°C). One exception is Warming 1, which must be warmed to 37°C before use. We strongly advice to read through all the steps of the vitrification/warming procedure before starting the procedure.
Preliminary steps:
- For thawing of 4-cell embryos till blastocyst, the following media are NOT required:
GM501 GentleVit Thaw Medium 5
- In a 6-well dish, fill the first well with 500-800μl of Thaw Medium 1, the second with Thaw Medium 2, the third with Thaw Medium 3, the fourth with Thaw Medium 4, the fifth with Thaw Medium 5 (only for zygotes) and the last with Thaw Medium 6.
Thawing protocol:
- In the first warming step, ensure homogeneous temperature by swirling the straw gently in GT1.
- Embryos are sequentially exposed to the following media:
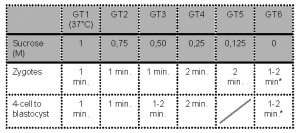
* Note: Wash for 1-2 min before transfer to culture medium.
Precautions and warnings:
- Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot totally be excluded. This also applies to unknown or emerging viruses and other pathogens. There are no reports of proven virus transmissions with albumin, manufactured to European Pharmacopoeia specifications by established processes.
- Therefore, handle all specimens as if capable of transmitting HIV or hepatitis. Always wear protective clothing when handling specimens.
- Always work under strict hygienic conditions (LAF-bench ISO Class 5) to avoid possible contamination.
- Only for the intended use.
- The long term safety of oocyte and embryo vitrification on children born following this procedure is unknown.
- The user facility of this device is responsible for maintaining traceability of the product and must comply with national regulations regarding traceability, where applicable.
Pre-use checks:
- Do not use the product if it becomes discoloured, cloudy, or shows any evidence of microbial contamination.
- Do not use the product if seal of the container is opened or defective when the product is delivered.
Storage instructions and stability:
- The shelf life is 12 month from time of manufacture.
- Store between 2-8°C.
- Do not freeze before use.
- Keep away from (sun) light.
- The product can be used safely up to 7 days after opening, when sterile conditions are maintained and the products are stored at 2-8°C.
- Stable after transport (max. 5 days) at elevated temperature (≤ 37°C).
- Content cannot be re-sterilized after opening.
- Do not use after expiry date.

