GM501 Gradients CE marking according to new MDR
Valued customer,
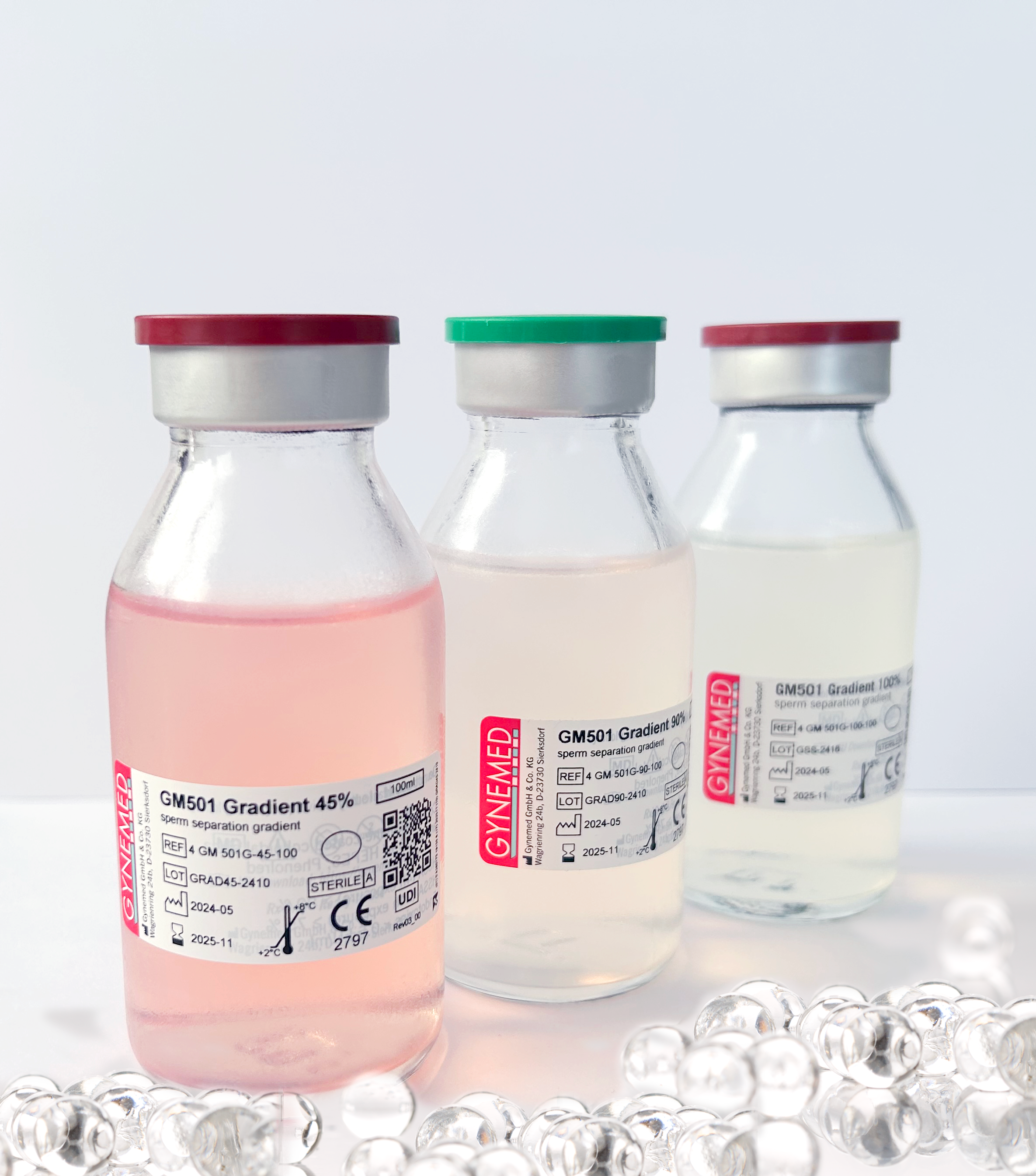
We are very pleased to announce that GM501 Gradient 45%/ 90%/ 100% is now CE-marked in conformity with the new European Medical Device Regulation (MDR, 2014/745.
CE marking of the product according to the MDR does result in the following changes:
- Use of 3-layered labels
- New version of instructions for use (45/90 Rev03_00) (100 Rev03_00)
- New version of MSDS (45/90 Rev11_00) (100 Rev10_00)
- Certificate
If additional information or additional registration actions in your jurisdiction(s) are required, please send your question to [email protected].

